Isoelectronic species - Isoelectronic Species Chemistry Tutorial
What are isoelectronic species?
Often, these chemical species have similar electronic properties as well.
The increase in atomic size going down a column is also due to electron shielding, but the situation is more complex because the principal quantum number n is not constant.
The following infographic summarizes the differences between isoelectronic and isosteres in tabular form for side by side comparison.
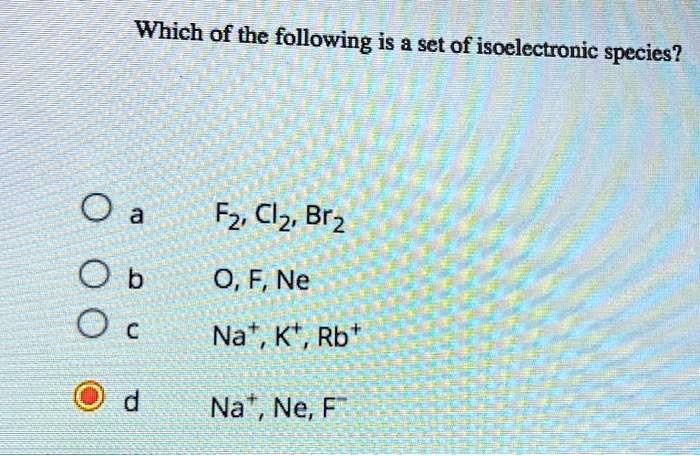
What is an isoelectronic species?
Then, applying the same arithmetic, find another species that has the same number.
Similarly, as we proceed across the row, the increasing nuclear charge is not effectively neutralized by the electrons being added to the 2 s and 2 p orbitals.
Consequently, beryllium is significantly smaller than lithium.
- Related articles
2022 qa1.fuse.tv
















:strip_icc():format(jpeg)/kly-media-production/medias/3250844/original/072682500_1601264286-pexels-george-desipris-753619.jpg)














