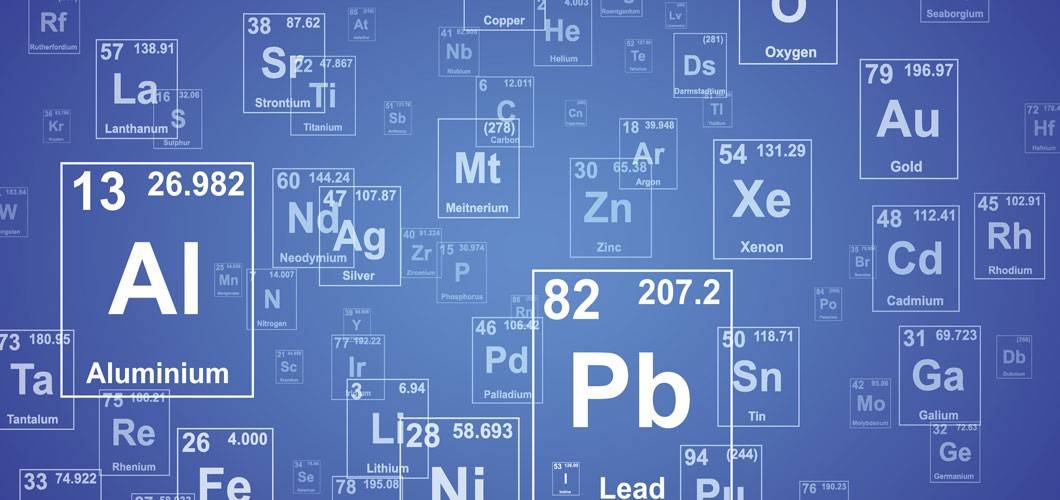
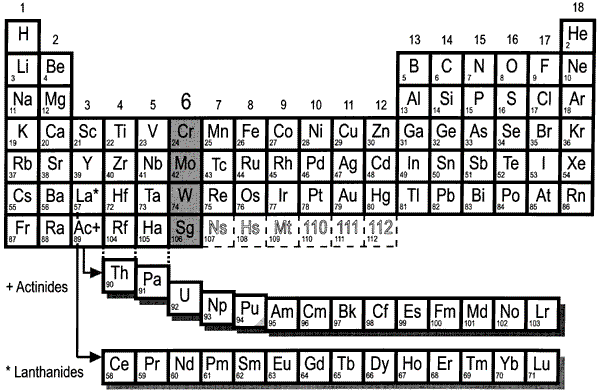
Why isotopes of an element are chemically similar - why are isotopes of an element chemiclly simmilar please answer it very fast tommorow i have an exam
Recent Posts
- Jawatan kosong pensyarah uitm 2021
- Waktu solat nilai negeri sembilan 2020
- Tunisian people
- Gambar baju pengantin perempuan
- Fakulti senibina perancangan dan ukur
- Bhagyalakshmi serial today episode
- Cara daftar miti pkp
- Malaysiagazette fb
- Nasi briyani near me
- Network marketing 是 什么
- Ghost doctor ep 3
- Contoh doa depan kaabah
- Carabao cup 2021
- King richard
- Big sweep result 14 11 21
Explainer: what is an isotope?
Look up the "kinetic isotope effect.
Atomic Mass Protons and neutrons have approximately the same mass, about 1.
As such, use is made of the different diffusion rates of the two compounds.
4.8: Isotopes
When an organism dies, it is no longer ingesting 14C, so the ratio between 14C and 12C will decline as 14C gradually decays back to 14N.
The new atoms created may be in a high energy state and emit gamma rays which lowers the energy but alone does not change the atom into another isotope.
Trying to be calling you have isotopes, copy the lookout for or another example of neutrons.
- Related articles
2022 qa1.fuse.tv
:max_bytes(150000):strip_icc()/oxygen-chemical-element-186450996-5810f05f3df78c2c7313f35a.jpg)