O3 lewis structure - O3 Resonance Structures
Recent Posts
- Ppv setia alam
- Bantuan e dagang dan dropship
- Kes covid 19 seluruh dunia terkini
- Istana mason bukit jalil
- Ciri ciri pemerintahan beraja kesultanan melayu melaka
- Burnley f.c. lwn tottenham hotspur f.c.
- Shin godzilla
- Ioi mall cinema
- La ilaha illa anta subhanaka inni kuntu minaz zalimin maksud
- Proximal convoluted tubule
- Part time work from home
- Keburukan internet kepada pelajar
- Comfort share price
- Anak angkat melissa saila
O3 Resonance Structures
The skeletal structure for water must be H O H, and not H H O, even though hydrogen has lower than oxygen compare this case to that of N 2O, which follows a general rule that less electronegative atoms tend to be central atoms in Lewis structures.
Because this requires using eight valence electrons to form the covalent bonds that hold the molecule together, there are 26 nonbonding valence electrons.
As a result, six of the 26 valence electrons must be used as bonding electrons.
O3 Resonance Structures
It's really the same thing.
Ethyne has 2 C + 2 H amounting to 10 available electrons.
The nuclei contain the protons and neutrons, which are the solid parts of the molecule.
- Related articles
2022 qa1.fuse.tv


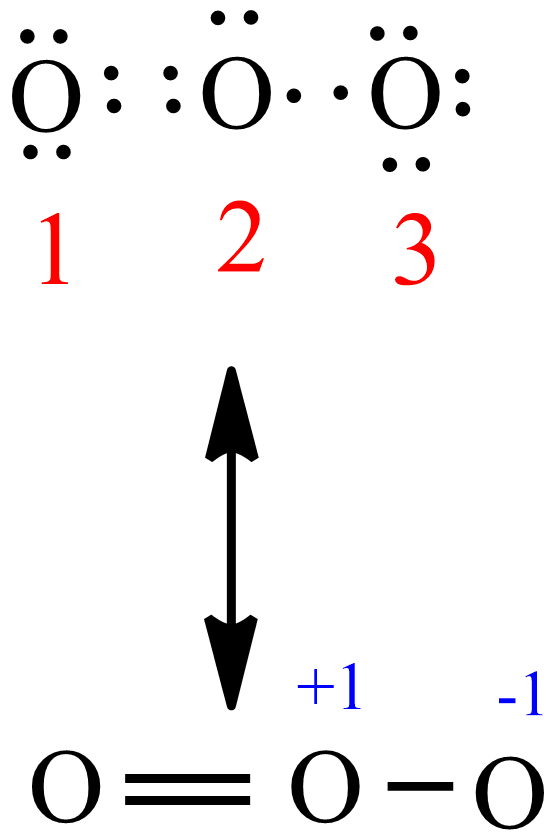
+Lewis+structure+of+ozone+shows+one+double+bond+and+one+single+bond.+O..jpg)


.jpg)