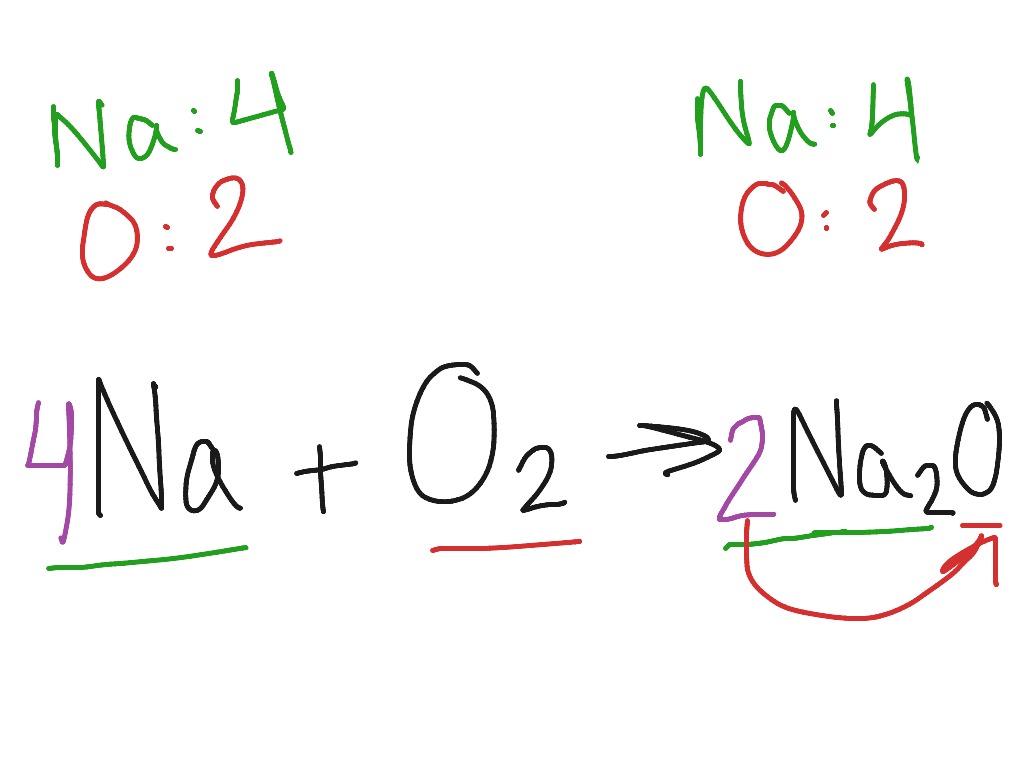Sodium oxide - Sodium Oxide: Formula, Properties, Risks and Uses
Sodium oxide Definition & Meaning
Moreover, many chemicals, particularly in the E400—499 range, have a variety of purposes.
But lithium is present just above sodium.
A single oxygen atom makes up the oxidization, and it has a couple of ionic bonds with two sodium atoms.
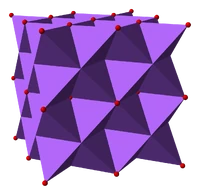
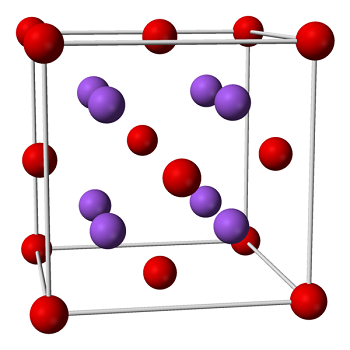
Sodium Oxide
Occurrence: Sodium oxide is not found in nature.
Formation of bond in Na2O, etc.
Hence, the oxygen nonmetal gains the 2 electrons to achieve the stable form.
- Related articles
2022 qa1.fuse.tv