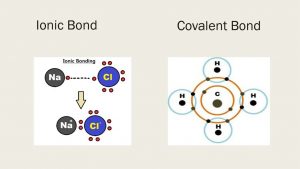
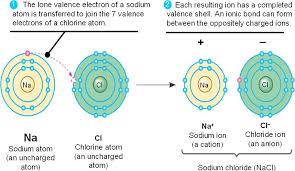
Ionic bond - Chemical Bonds: Types of Bonds in Chemistry, Examples & More

Recent Posts
- Mortal housewife
- Manchester city f.c. lwn tottenham hotspur f.c.
- Exodus meaning
- Digi centre near me
- Bcw advance bahaya
- Senarai barangan my kasih
- Agape love
- Tinder swindler netflix
- Javtiful
- The medium movie cast
- Maksud fyp dalam bahasa melayu
- King affection
- Arkadia desa park city food
- Bantuan subsidi kerajaan
- Permainan kategori serangan
- Semak semula keputusan stpm
Ionic bonds (video)
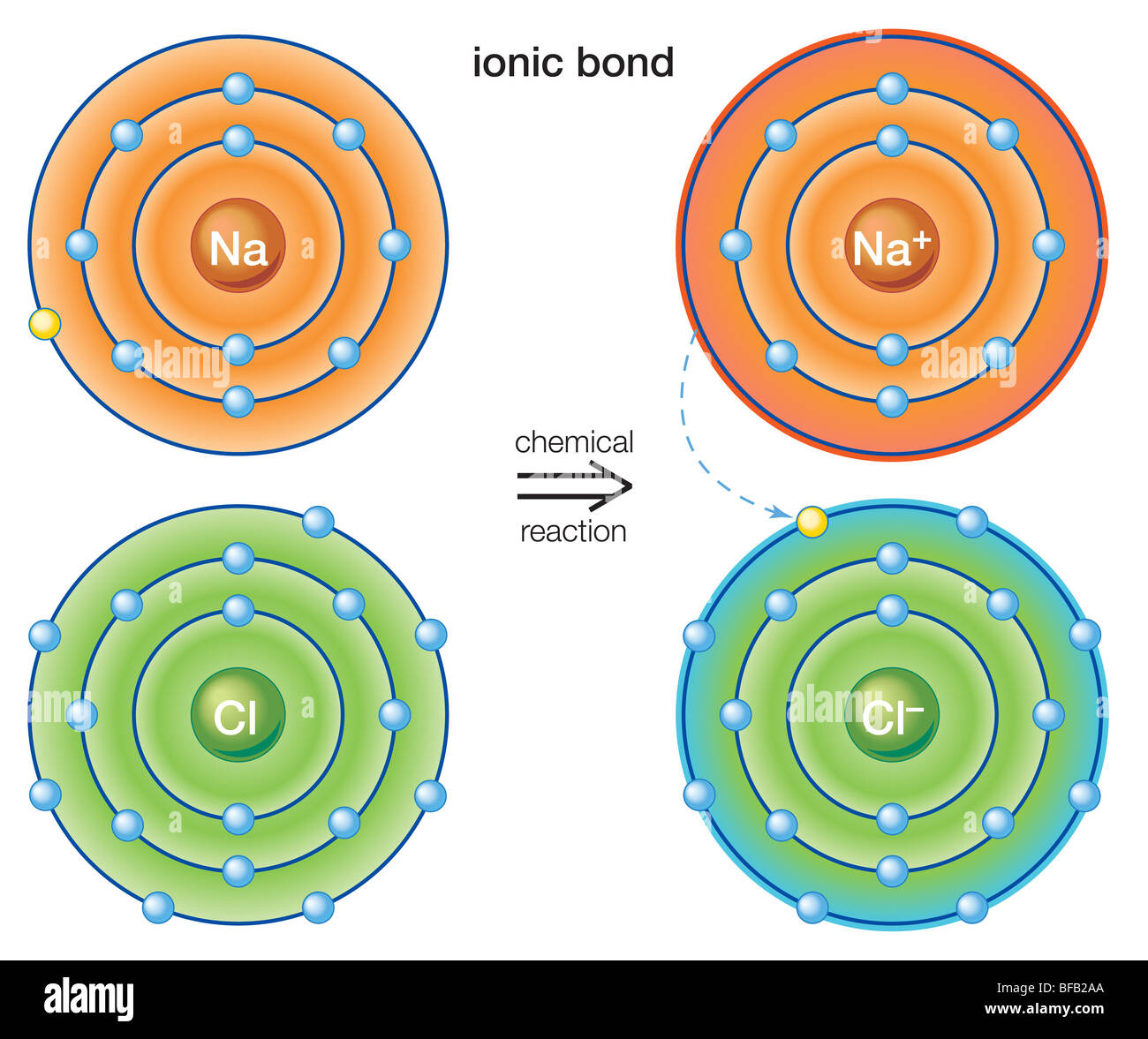
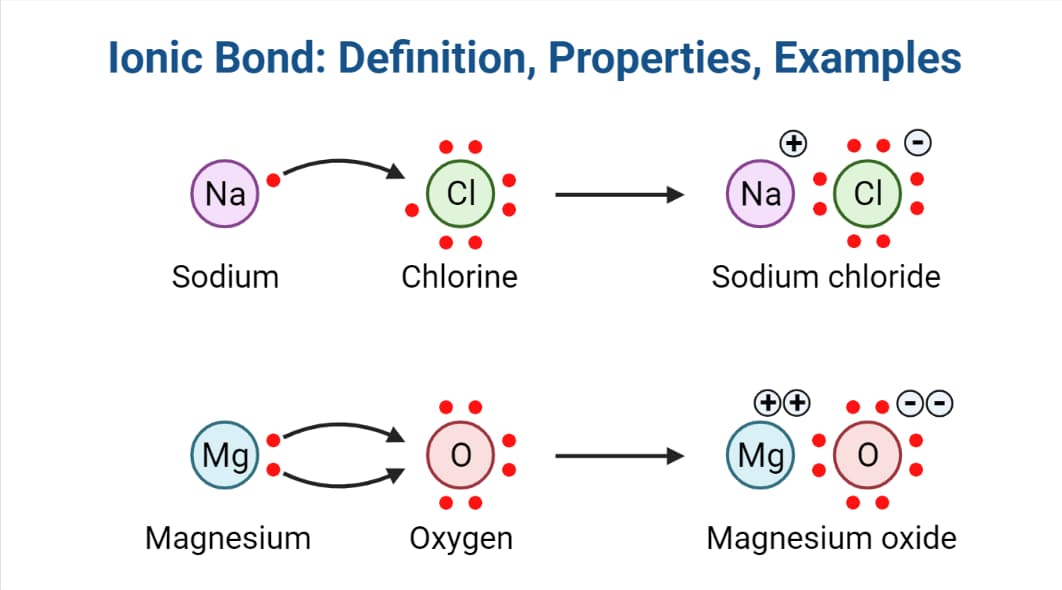
Comparing the two relative forces of electrostatic attraction that you calculated, you can conclude that ionic bonding is considerably stronger in magnesium oxide.
Metallic Bond A metallic bond is a chemical bond, in which the atoms do not share or exchange electrons to bond together.
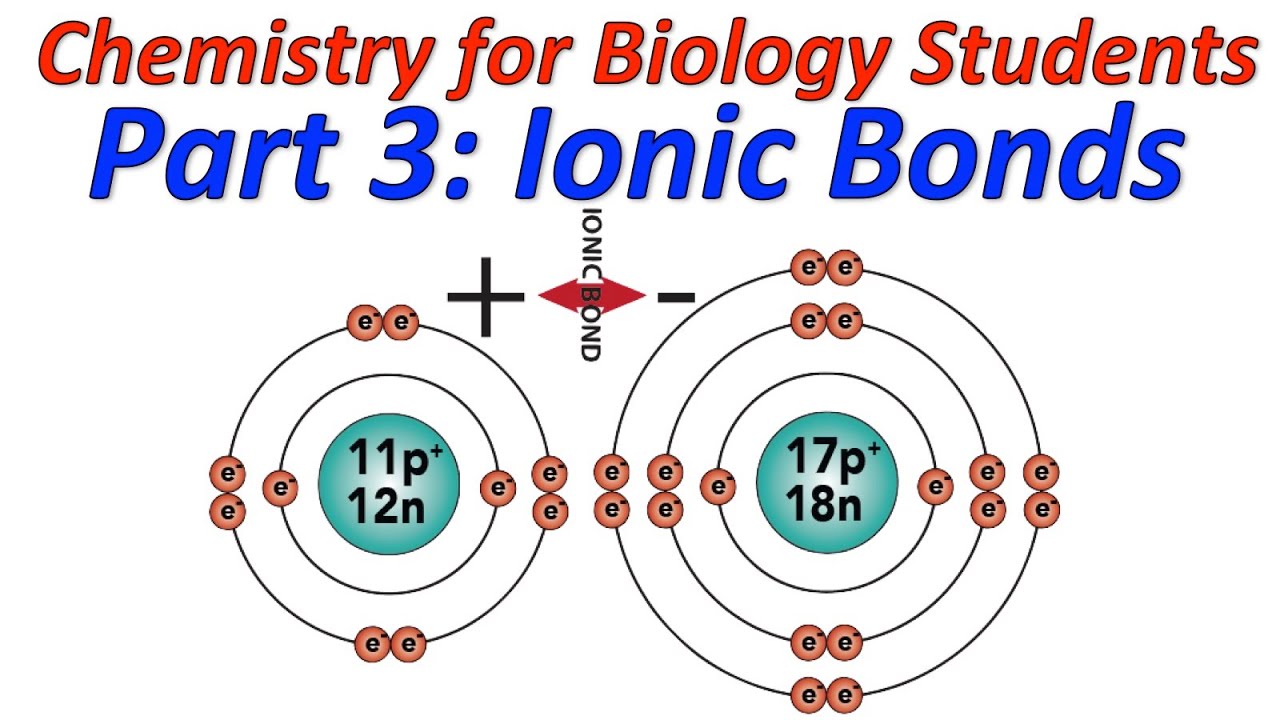
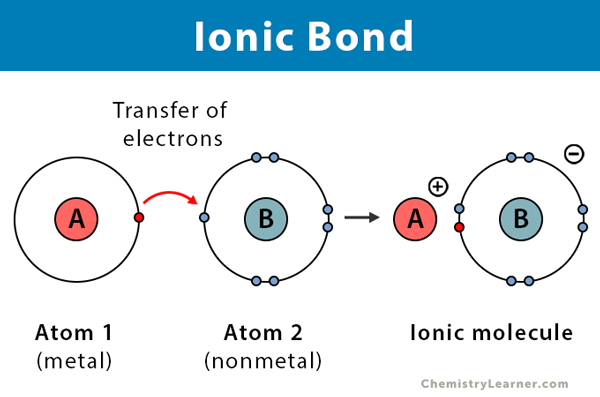
However, the action of the anion's accepting the cation's valence electrons and the subsequent attraction of the ions to each other releases lattice energy and, thus, lowers the overall energy of the system.
- Related articles
2022 qa1.fuse.tv


(477).jpg)