Formal charge formula - Formal Charge Uses, Formula, and Examples
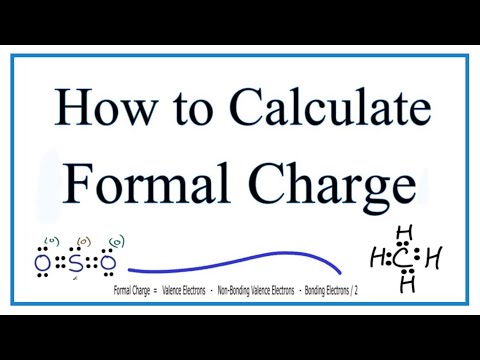
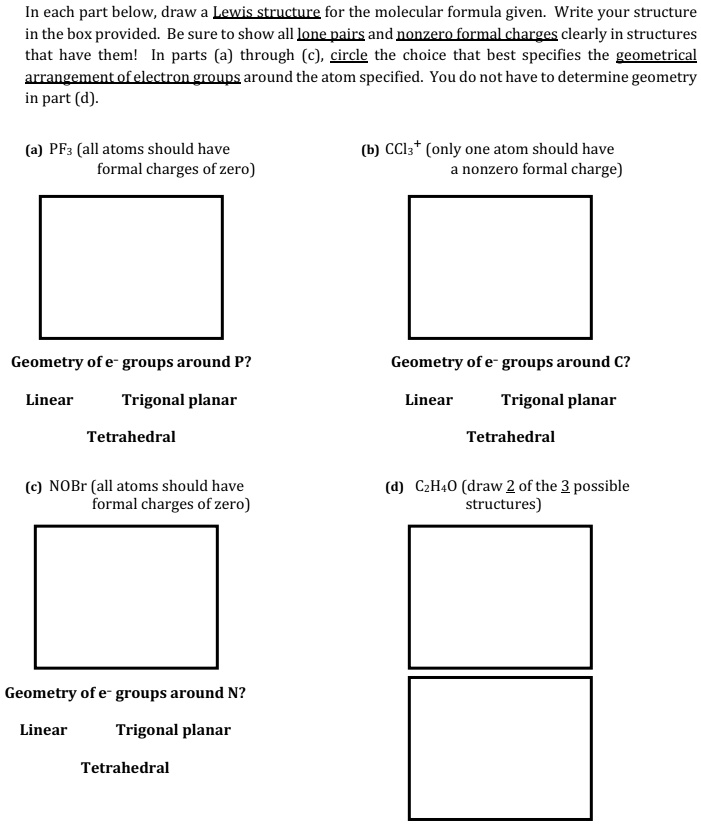
How To Calculate Formal Charge Formula
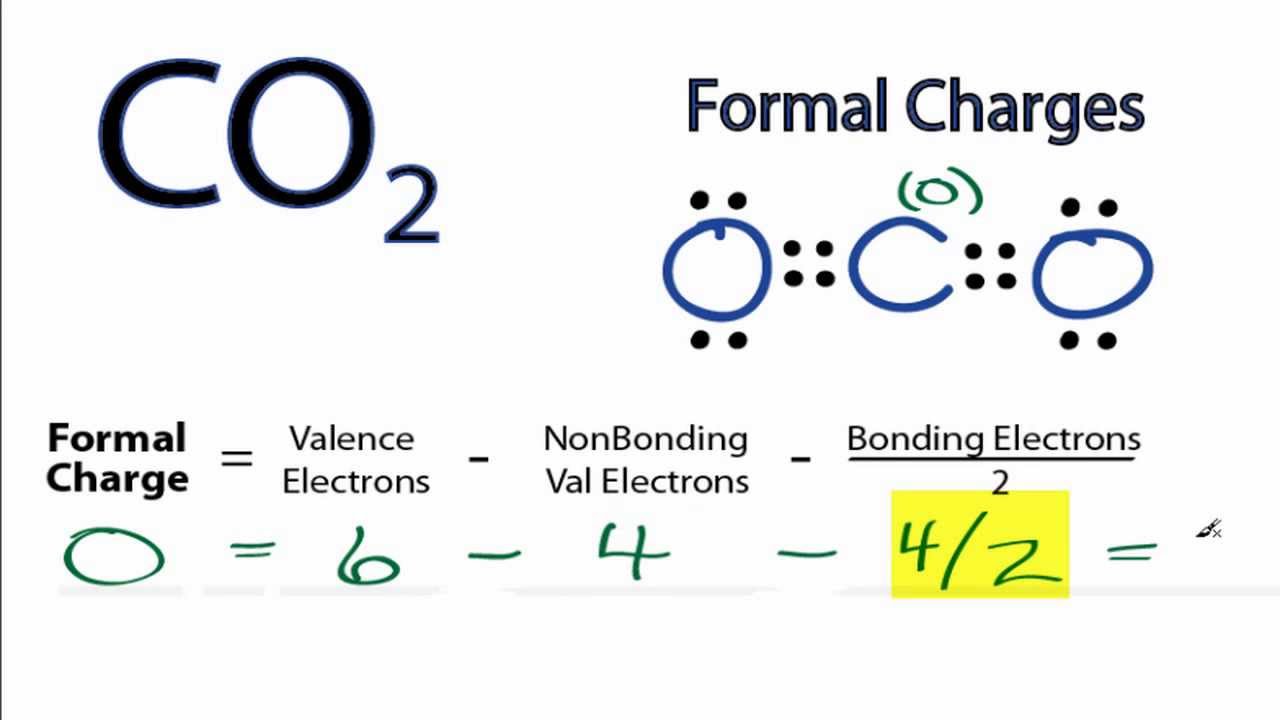
The fact that carbon dioxide does not have a superscript that pertains to charge means that the overall charge should be equal to zero.
So, the hydrogen is more electronegative not by much but still and will polarize the bond.
That denotes that I only have a partial negative charge on each of the hydrogens.
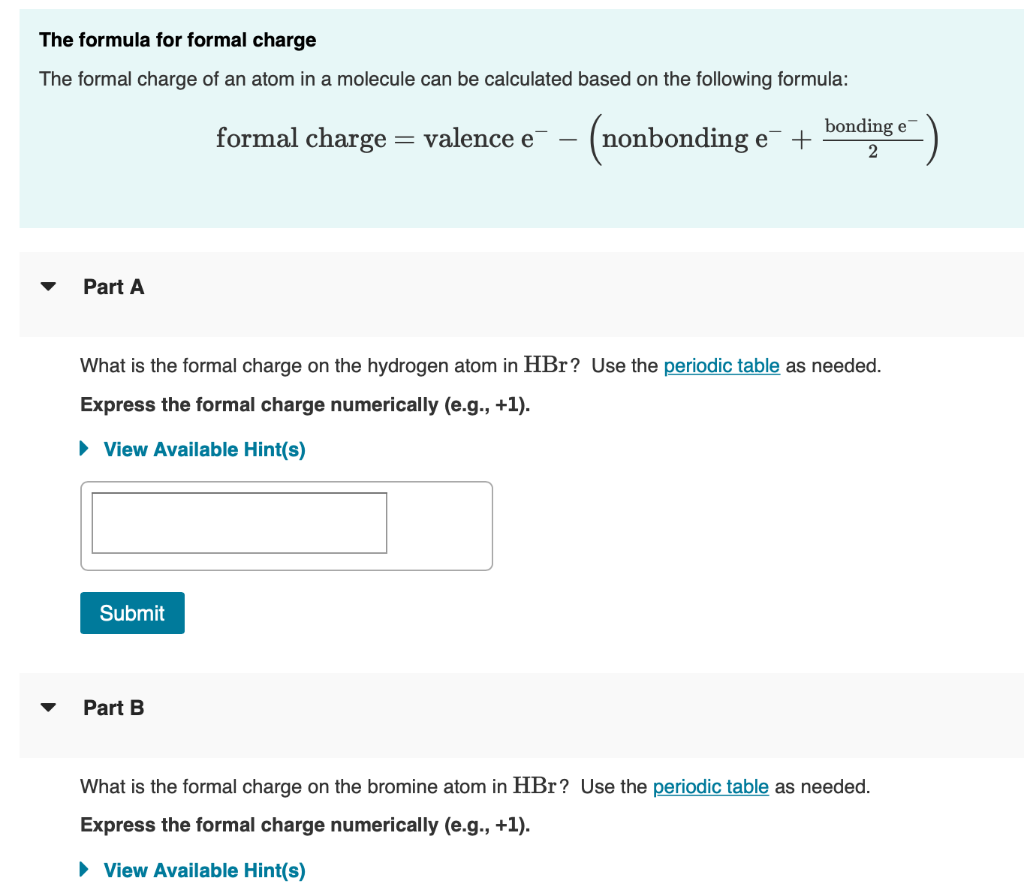
How to Calculate Formal Charge?
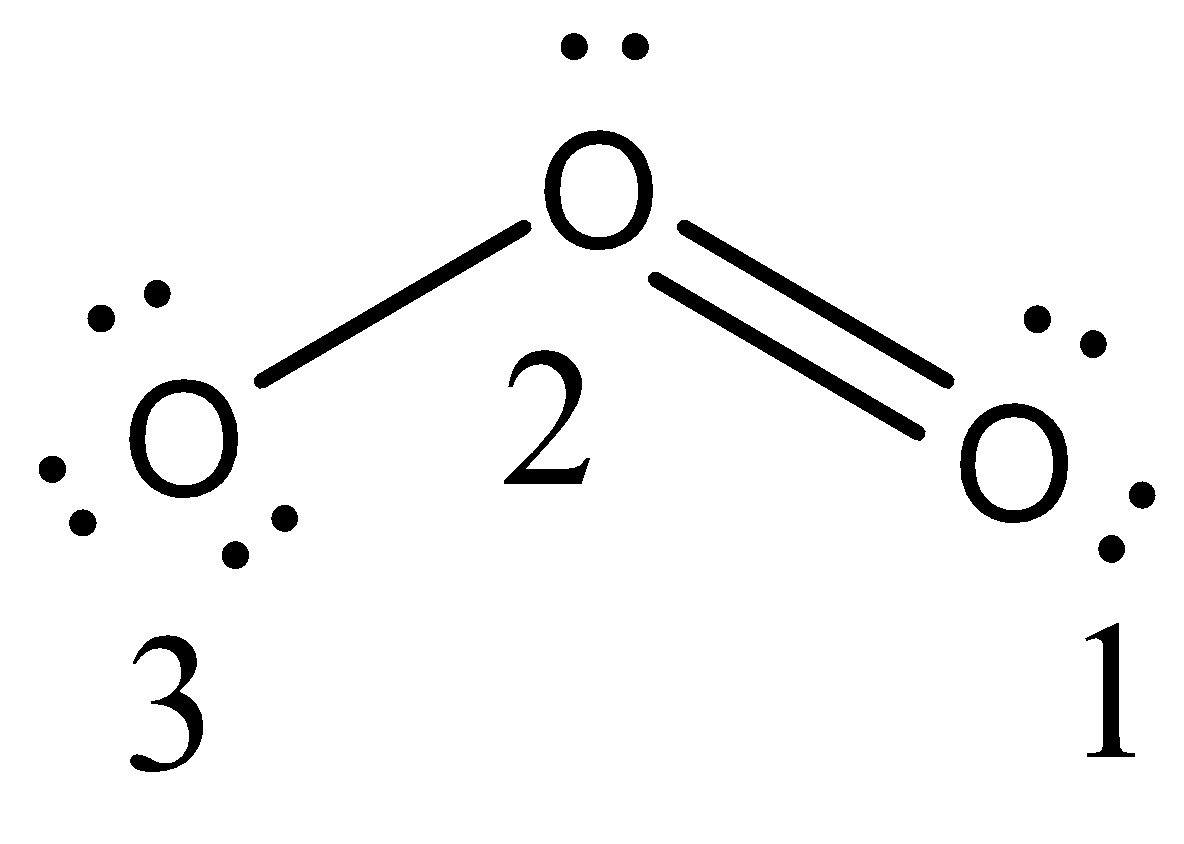
All oxygen atoms, however, are equivalent, and the double bond could form from any one of the three atoms.
Try using both the formula and the patterns shown above to make the formal charge assignments.
Double-ended arrows are used to indicate that the structures are chemically equivalent.
2022 qa1.fuse.tv