Halide ion - Testing for Halide Ions

Recent Posts
- Penaja ajl 35
- Semakan status rayuan bpr 2021
- Mercedes g class
- Habib jufri
- Tadika pasti
- Your lie in april live action
- Persephone pink
- Anita mui
- Marrybrown klebang ipoh
- Oki setiana dewi dulu
- Harga hati manusia
- Xxl scrunchie
- Keputusan penuh prn melaka 2021
- Drama sabarlah duhai hati full episode
- Rumput jarum emas
- Olympic rtm malaysia
Halide
Thus fluorine attracts an extra electron to complete its outer shell, most strongly, and is therefore the most powerful oxidising agent in the Group.
The fluoride and chloride ions aren't strong enough reducing agents to reduce the sulphuric acid.
Metal halide lamps are also commonly used in greenhouses or in rainy climates to supplement natural sunlight.
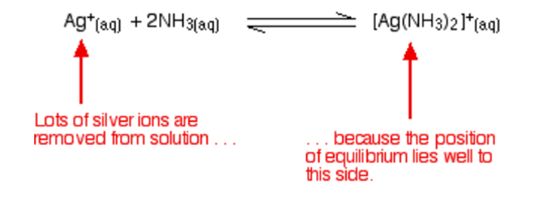
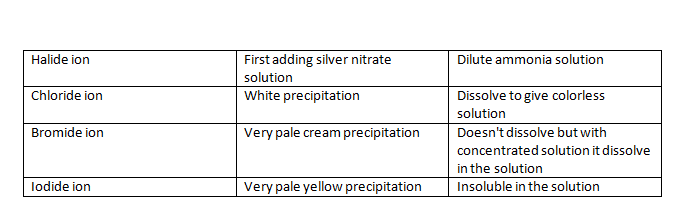
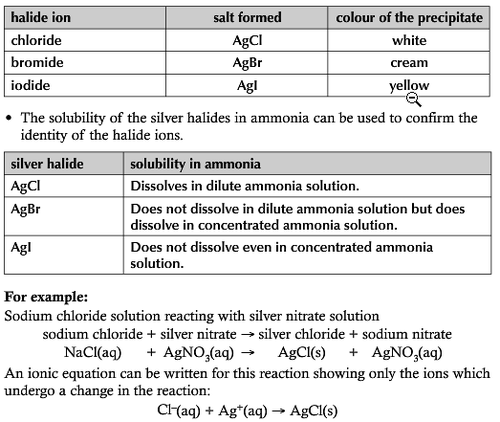
Testing for halide ions
This is due to the small size of the fluoride ion, which means that the positive and negative ions are very close together and so strongly attracted to each other.
The potassium bromide solution gradually turns pale yellow due to the formation of bromine.
Fluorides of are sparingly soluble in water.
- Related articles
2022 qa1.fuse.tv





+Ag+NO3(aq)+Cl(s)+%2B.jpg)