Cansino vaccine origin - covid
Recent Posts
- Chicken rice shop mesra mall
- Facebook login
- Faktor perdana bagi 60
- Quizizz sejarah tingkatan 3 bab 1
- Pgmall
- Klinik perkasa
- Jpn pekan
- Keracunan makan
- Mcplus login
- Sayaka kanda voice actor
- Google workspace for education
- Umur ustaz wadi anuar
- Pemilih warna
- Men hairstyle 2022
- N&d cat food
- Zhongli artifacts
- Link kehadiran
covid
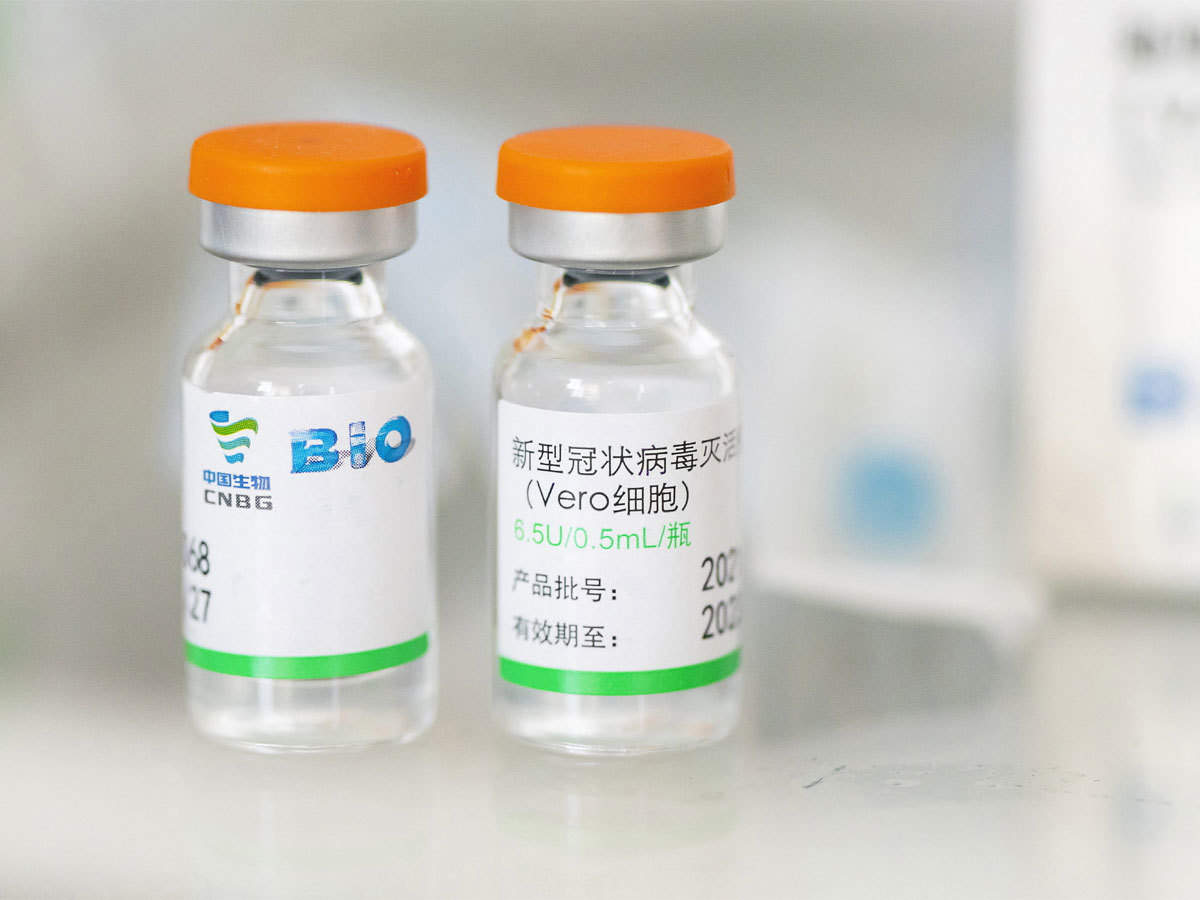
Referred to as Ad5-nCoV, the vaccine candidate received Chinese regulatory approval earlier this year, allowing CanSino Biologics to move ahead with human clinical trials in China.
This advertisement has not loaded yet, but your article continues below.
But, there can be problems too; these vaccines require two doses to be administered, while a small minority of patients can suffer chronic reactions to such vaccines and even develop the disease, something that the CanSino vaccine is less prone to induce.
Convidecia
In February 2021, Mexico approved the vaccine for emergency use.
Because trials in Argentina found that a second shot of Moderna after the first Sputnik shot was superior to 2-shot Sputnik V.
In July, 2021, Cansino said it would begin combination trials with a dose of followed by a dose of Convidecia.
- Related articles
2022 qa1.fuse.tv

/cdn.vox-cdn.com/uploads/chorus_asset/file/21874714/GettyImages_1228369332.jpg)



![Vaccine origin cansino The National Vaccine origin cansino [PDF] Vaccine](https://www.aljazeera.com/wp-content/uploads/2021/02/Interactive-covid-vaccine-rollout_Feb-3-2021_2ai-01.jpg?w=770&resize=770%2C770)